On 8 June 2011, the European Parliament and Council Directive 2011/62/EU – Falsified Medicines Directive – was published, obliging Member States to implement a medicines verification system from 9 February 2019. The obligation was placed on the states, but the development and on-going management was to be financed by the private sector (by pharmaceutical manufacturers).
Dear reader
You are holding the first yearbook of REKS (Estonian Medicines Verification Organisation) which provides an overview of the most important events starting from the establishment of the system and the organisation up to the end of 2018.
The yearbook also covers the introduction process of the medicines verification system, including the development of the system by activities of the partners and related organisations of REKS in Estonia and Europe.
REKS is the Medicines Verification Organisation, founded in 26 October 2016.
REKS was founded by the Association of Pharmaceutical Manufacturers of Estonia (RTL), the Estonian Association of Pharmaceutical Wholesalers, and the Estonian Pharmacies Association. The work of the organisation is managed and organised by a six-member Supervisory Board chaired by Riho Tapfer as of 26 October 2016. The Estonian Society of Hospital Pharmacists is also represented in the Supervisory Board via the Estonian Hospitals Association. On a daily basis, the activities of REKS are managed by Raul Mill. As of 2018, REKS employs three people. REKS would like to thank all the founders and members of the Supervisory Board for their substantial and significant contribution to the system’s development. Special thanks also goes to the members of RTL whose loans and advances made it possible to build the system.

- AbbVie Biopharmaceuticals GmbH Eesti filiaal
- AstraZeneca AB
- Bayer OÜ
- BGP Products Switzerland GmbH Estonian affiliate
- Boehringer Ingelheim RCV GmbH & Co KG Eesti filiaal
- Eli Lilly (Suisse) S.A, Rep Office Estonia
- Gedeon Richter Plc
- GlaxoSmithKline Eesti OÜ
- Lundbeck Eesti AS
- MSD HUMAN HEALTH HOLDING B.V.
- Novartis Pharma Services inc Eesti filiaal
- Novo Nordisk A/S
- OÜ Berlin-Chemie Menarini Eesti
- Pfizer Estonia OÜ
- Roche Eesti OÜ
- Sandoz Pharmaceuticals d.d
- Sanofi-Aventis Estonia OÜ
- Takeda Pharma AS
- UAB "JOHNSON & JOHNSON" Eesti filiaal
- UAB Sicor Biotech Eesti filiaal
History

A Memorandum of Understanding between the Efpia, PGEU and GIRP was signed.

The Memorandum of Understanding was signed with other stakeholders.
The Directive was transposed into the legislation of the Member States.
The design of the European Medicines Verification System (EMVS) was developed.
The developer of the system’s central database (EU-HUB) was selected.
On 13 February, the European Medicines Verification Organisation (EMVO) was founded. The EMVO is a non-profit organisation that manages the central HUB and coordinates the activities of the NMVOs (National Medicines Verification Organisations). The EMVO was founded by the Efpia, MfE, GIRP, EAEPC, and PGEU.
Commission Delegated Regulation (EU) 2016/161 of supplementing Directive 2001/83/EC of the European Parliament and of the Council by laying down detailed rules for the safety features appearing on the packaging of medicinal products for human use was issued on 3 October. It is a directly applicable regulation that must be implemented by the Member States. The regulation stipulated that it would apply as of 9 February 2019.

In September, the Memorandum of Understanding was signed by wholesalers, pharmacies and pharmaceutical companies.

Pursuant to the established requirements, packaging the medicinal products will carry a safety feature, the data of which would be forwarded to the information system to be created.

In addition, the "tamper evidence" security element shall be mandatory for packaging.
Delegated acts which allow 36 months for setting up the system were published on 9 February. It is a directly applicable regulation that must be implemented by the Member States which join the system.
Pursuant to the requirements of the directive and the regulation, it is up to marketing authorisation holders in cooperation with wholesalers and pharmacies of each Member State to establish a non-profit organisation (the NMVO) and set up a national database of the safety features of medicinal products.
The Falsified Medicines Directive (FMD) was to be fully implemented by 09/02/2019.
The aim is to protect patients in the legal supply chain from falsified medicinal products
The content is aimed at the establishing of the European Medicines Verification System
-
July 2011
Publication of the falsified medicines directive
-
-
09.02.2016
Publication of delegated acts
-
-
09.02.2019
Full implementation
Non-compliance meant that medicinal products could not be legally supplied to patients.
On 26 October, the Medicines Verification Organisation, i.e. REKS, was founded.
A significant decision which later proved successful was that REKS should be founded by all the parties relevant to the implementation of the system – pharmaceutical manufacturers, wholesalers and pharmacies, including hospital pharmacies.
The parties have the following obligations:
Manufacturers shall develop new secure packages for medicinal products and each packaging shall be provided with a unique safety feature and anti-opening device.
Wholesalers and pharmacists must have the technical capability to scan the 2D code from to the packaging and have the ability to verify it in a national database that will be created.
It is up to the Member States to advise and supervise the project, as well as to bring the legislation into conformity with the directive and the regulation.
Negotiations with developers started (Blueprint is the technical solution).
The development of the system is financed by loans and advances guaranteed by members of the RTL in Estonia. In addition, the founders will make their own contributions during the development period of the system.
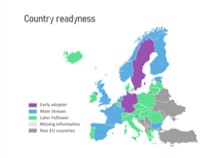
The preparedness of countries to implement the system varies. The EMVO maps the preparedness of countries regarding the implementation of a medicine verification system.
A project steering group was formed.
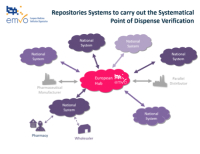
The architecture of the system foresees that data will be uploaded to national repositories via the central part of the database, i.e. the EU-HUB. All trans-national data exchange is also performed via the EU- HUB.
32 countries joined the system.
It was estimated that 2,500 pharmaceutical manufacturers would be interfaced with the system and approximately 10.5 billion pharmaceutical packages subject to serialisation would have to be redesigned.
The system would be developed by two developers: Arvato, which is part of the Bertelsmann Group, and Solidsoft. All countries chose between these two developers.
Arvato would start developing the Estonian system. A total of 16 countries chose Arvato as their development partner. An agreement with Arvato was entered into on 29 December 2017, which is also the official start date for the development of the Estonian system.

In November, an international seminar organised by the RTL that was dedicated to the implementation of FMD was held in the Tallinn Creative Hub (Kultuurikatel).

The website of REKS along with a new visual identity was introduced before the conference.
In November, a seminar for the future users of the system was held in Tallinn with Arvato.
In December, a serialisation symposium organised by Arvato took place in Berlin. In addition to presentations, the symposium also included workshops on key issues in system implementation.
And then it was 2018
The system’s architecture and conceptual structure were in place. The system consists of a central EU-HUB that pharmaceutical manufacturers / marketing authorisation holders use to upload data on the safety features of pharmaceutical packages.
The EU-HUB is connected to the respective repository of each country, where data are forwarded to based upon which market/destination they are assigned to. Information exchange between the repositories of different countries will also take place via the EU-HUB if necessary.

A manufacturer or their representative will upload data on the safety feature of a medicinal product’s packaging to the central EU-HUB and from there the packaging data shall be forwarded to the database of the country of destination. Before dispensing the packaging, the end-user shall verify the authenticity of the packaging by scanning it in or manually comparing it with the repository of the country of destination. The process is the same in all countries.

The REKS team was increased to three members and the office was moved to the Fahle building on Tartu maantee.
In January, Arvato organised a meeting for its clients in Vienna to discuss the action plan and development details for the coming year.
During the year, project management meetings organised by the EMVO were held to provide information to all countries that joined the system. In addition, meetings on key issues relating to the structure of the system organised jointly by the EMVO and Efpia were held in Brussels.

On a monthly basis, the EMVO provided an overview of the implementation of the system by each Member State.
An agreement was entered into with the EMVO on 18 February. It is a cooperation agreement which was only entered into for the duration of the development phase, i.e. until the system was implemented.

In March, REKS organised information days in Tallinn and Tartu for all parties to the system. The activities necessary for implementing the FMD and interfacing with the system were introduced to participants during the information days. The project’s timetable was presented, a brief overview of what was going on was provided, and issues and problems that had arisen were discussed.

UATs (user acceptance tests) were conducted in March. The tests were conducted in Arvato’s development centre in Gütersloh, Germany in cooperation between Arvato and the NMVO of Finland (FiMVO). There were also representatives of NMVOs of other countries that would use Arvato's solution.

Arvato would provide a portal solution for pharmacies and software developers of wholesalers that they can use to access the technical documentation required for the interface. Later, Baseline testing aimed at end-users could also be completed in the same environment.
By the beginning of 2018, 16 countries had signed a development agreement with Arvato. The cooperation between the countries was close. Regular weekly Skype meetings as well as monthly face-to-face meetings were held. Issues related to development were discussed and experiences in building the system were shared in the meetings.
REKS brought the implementation of the quality system into conformity to the extent required by the EMVO and also underwent the evaluation required in this respect. The implementation of the quality system was a prerequisite for joining the Estonian database test environment with the EU-HUB.
The Ministry of Social Affairs and the State Agency of Medicines prepared amendments to the Medicinal Products Act. In addition to the training organised by REKS, the State Agency of Medicines also covered issues related to the joining of the NMVOs in its seminars.
On a quarterly basis, REKS sends a newsletter to all parties on the current status and timeliness of the system development, including on the development of information systems for related end-users and preparedness of organisations.

In April, the Estonian test environment (IQE) was joined with the EU-HUB. The parties to the Estonian system could start with testing.
In April, the Supervisory Council of REKS approved the financial model for 2019. This was preceded by a series of meetings with marketing authorisation holders, where proposals and concerns about fees were heard and various options for financing models were discussed. When developing the financial model, priority would be given to ensuring the availability of medicinal products on the Estonian market where consideration regarding the ability of marketing authorisation holders with a smaller turnover to interface with the system also had to be given.
Estonia would introduce a so-called adjusted flat fee model which takes into account the turnover-based exemptions of companies. One important aspect is that no system interfacing fees – which are used by several countries – would be imposed on marketing authorisation holders.
In May, an information day for developers was organised in Tallinn and Arvato also attended. Technical nuances required for interfacing end-users, including the use of the necessary certificates in the computers of end-users were discussed in the information day.
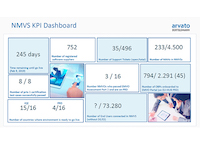
Progress in system development.
In June, REKS started entering into agreements with marketing authorisation holders. This was a lengthy process that lasted until the end of the year and continued into 2019.
In June, REKS underwent an evaluation of the second EMVO quality system which was the prerequisite for joining Estonia’s so-called live environment with the EU-HUB (PROD). The integration of the production environment was planned for 29 July 2018. However, on 30 June, i.e. a month ahead of schedule, the Estonian production environment had already been joined with the EU-HUB and was open for uploading data by marketing authorisation holders. Data for 16 products from one batch were uploaded in July 2018 and by 1 September 2018, data on 269 batches and 190 product names had been added to the environment.
In June, the testing solution for Baseline was completed. The first tests were to be conducted in August. The first one to undergo testing was the software NOOM Pharmacy Version (developed by Apteekide Infotehnoloogia OY). Those who passed the test would be certified accordingly.

Verification of medicines at a pharmacy.
REKS has developed a system for managing user accounts. Pursuant to the technical requirements of the system, each user must download the certificate and install it on their computer. REKS creates accounts for the admin user of each end-user. In addition to the manuals provided, REKS also offers online user support via computer and telephone to streamline the certificate installation.
In July, REKS started entering into agreements with end-users for using the system. In total, over 300 agreements were entered into with marketing authorisation holders and end-users (legal entities), allowing more than 700 parties to interface with the system.
The first end-user interfaced with the system's test environment in August and with the production environment in November.
In November REKS sent out additional newsletters to all parties to the system.
In the second half of 2018, several memos were issued and calls were made to contracting partners to ensure timely interface with all users, marketing authorisation holders, pharmacies, hospital pharmacies, and wholesalers.
In December, REKS started preparing for the seminar "Implementation of the FMD on 9 February 2019" held in January 2019.

In December, the Riigikogu passed the act that amends the Medicinal Products Act.
Main keywords for 2018
-
By the end of the first semester, the live environment of REKS was completed.
-
90%
A total of 90% of end-users had entered into an agreement with REKS.
-
886
Data on 886 batches had been uploaded to the system.
-
1232
Data on 1,232 product names had been uploaded to the system.
Addendum
,,We would like to thank all parties for their contribution to developing the system. We would like to thank the pharmaceutical manufacturers who redesigned their production lines to supply new packaging with security features and end-users who made the necessary developments to interface with the system and redesigned their work processes. We managed to build the entire system in 36 months. It is also noteworthy that all EU countries, with a few exceptions, plus Norway, Switzerland and Liechtenstein, introduced the system on time, on 9 February at 00:00 CET. There are only a few systems that have been successfully implemented across borders this punctually in the whole of Europe. The conceptual structure of the system is certainly worth mentioning – the system was funded, developed and managed entirely by the private sector and its resources, although the Directive imposes an obligation on the Member States to implement it.
REKS is a very small organisation with only three employees and that is why it was initially difficult for us to realise how labour-intensive it would be to develop and deploy this system. The system we have created has a very wide scope. In the last two weeks of May 2019 alone, more than 100 million pharmaceutical packages were checked in Europe by the countries that joined the system, compared to nearly half a million in Estonia alone during the same period. The basis for this successful outcome has been very close and efficient cooperation between all the parties involved. These are extremely constructive, courteous and hardworking people who are focused on delivering results. For example, the 16 countries affiliated with Arvato have created a WhatsApp group for fast communication so that information could be exchanged 24/7. Another proof of efficient cooperation is the completion of the pan-European IT development in less than one year, which is more than remarkable for projects like this.
Finally, I would like to say in advance, as we are already in the year 2019, that when 2018 seemed, and indeed was, very concise and intense in terms of development, we still have a lot of work ahead. The use of the live system which has been in place since the beginning of 2019 has already indicated this, but with partners like you everything is achievable.”
– Raul Mill, REKS Chairman of the Board